Question 3
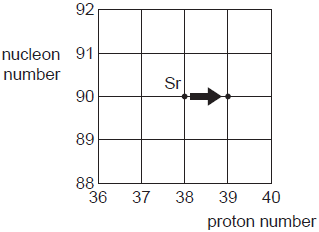
The radioactive decay of a strontium (Sr) nucleus is
represented in Fig. 7.1.
Fig. 7.1
(a) State whether Fig. 7.1 represents α-decay,
β-decay or γ-decay.
[1]
(b) One type of radioactive decay cannot be represented on
Fig. 7.1.
Identify this decay and explain why it cannot be
represented. [2]
Reference: Past Exam Paper – June 2007 Paper 2 Q7
Solution:
(a) β(-decay)
{Let the new element formed be X.
As the strontium (Sr) nucleus changes into X,
its proton number increases to 39 while its nucleon number remains the same.
9038Sr - - > 9039X
+ ???
For the equation to hold, the ‘proton number’
of the radiation should be -1 such that 39 + (-1) = 38
Its nucleon number should be zero.
This corresponds to a β-particle.}
(b)
γ-decay. This is because the nucleon number and proton number of the
nucleus do not change during this decay. (γ-radiation
is only a loss of energy)

No comments:
Post a Comment
If it's a past exam question, do not include links to the paper. Only the reference.
Comments will only be published after moderation